American biotechnology company Novavax, Inc. (NVAX) has filed for Emergency Use Authorization (EUA) of its COVID-19 vaccine with the Ministry of Health and Prevention (MoHaP) in the United Arab Emirates.
Following the news, shares are up almost 4% during pre-market trading at the time of writing.
NVX-CoV2373 Clinical Trials
The company’s COVID-19 vaccine, NVX-CoV2373 is a nanoparticle protein-based vaccine with Matrix-M™ adjuvant.
The submission included data from two pivotal Phase 3 clinical trials. The first trial, PREVENT-19, was conducted on 30,000 participants from the U.S. and Mexico. The study showed 100% protection against moderate and severe infection, 93.2% efficacy against the primary circulating variants of concern, and 90.4% overall efficacy.
The other trial conducted on 15,000 participants from the U.K. showed a 96.4% efficacy against the original virus, 86.3% efficacy against the Alpha (B.1.1.7) variant, and 89.7% efficacy overall.
Under both trials, the vaccine showed a reassuring safety and tolerability profile. Novavax and its manufacturing partner Serum Institute of India Pvt. Ltd. (SII) has received EUA for the vaccine in Indonesia and the Philippines. Moreover, both companies have filed for EUA in India and Emergency Use Listing with the World Health Organization.
Management Comments
President and CEO of Novavax, Stanley C. Erck, said, “The rapid emergence and continued spread of variants is a stark reminder that no one is safe until everyone is safe in the fight against COVID-19… We remain committed to delivering our vaccine, which is based on a proven, well-understood platform, to countries around the world as we anticipate that ongoing vaccination will be necessary over the long term to end the pandemic.”
See Analysts’ Top Stocks on TipRanks >>
Analysts’ View
Recently, B.Riley Financial analyst Mayank Mamtani maintained a Buy rating on the stock with a price target of $305, which implies a whopping 83.1% upside potential to current levels.
Commenting on Novavax’s success, Mamtani said, “The successful developments of NanoFlu, a quadrivalent seasonal influenza vaccine, and NVX-CoV2373, an adjuvanted nanoparticle vaccine against SARS-CoV-2, are critical to Novavax’s success. If the company fails or delays the development or commercialization of its product candidates, Novavax’s business prospects and operating results would suffer, and the stock price would likely decline.”
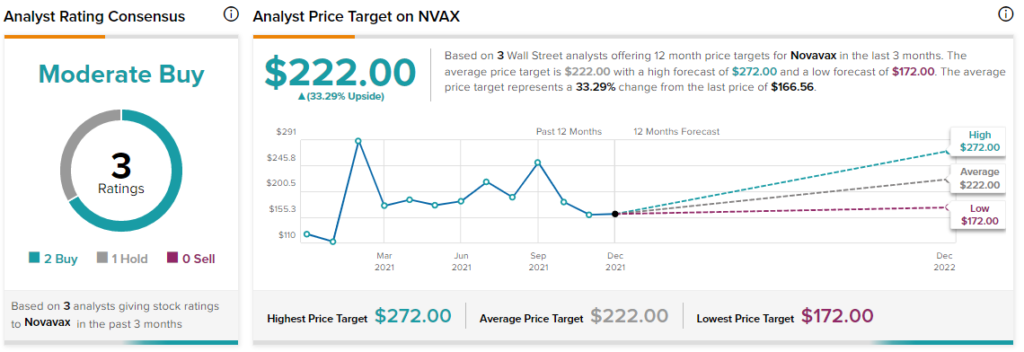
With 2 Buys and 1 Hold, the Novavax stock has a Moderate Buy consensus rating. The average Novavax price target of $222 implies 33.3% upside potential to current levels. Shares have gained 28.4% over the past year.
Related News:
CVS Impresses at Investor Day; Shares Reach New All-Time High
Roku Ends Battle with YouTube; Shares Surge 18%
Citi Halts Share Buyback Amid New Regulation