Moderna (MRNA) has announced that it has completed enrollment for its Phase 2 study of mRNA-1273, the company’s vaccine candidate against Covid-19. Shares are currently trading up 2% so far on Wednesday.
The clinical study will assess the safety, reactogenicity, and immunogenicity of 2 dose levels of mRNA-1273 SARS-COV-2 vaccine in around 600 adults 18 years of age or older. The patients will be split into those aged 18-55, and those above 55, and in each group two trial doses will be tested of 50mcg and 100mcg.
According to the trial record, the estimated completion date will be August 2021 with the estimated primary completion date expected for March 2021. This primary date refers to the date the last participant in the clinical study was examined or received an intervention to collect final data for the primary outcome measure.
The company also announced that the cohorts of older adults and elderly adults in the NIH-led Phase 1 study have completed enrollment. Results are expected to be published once available.
“We are committed to helping address this ongoing public health emergency and continue to focus on our Phase 3 study, which remains on track to start in July, less than seven months from the sequencing of the virus” said Tal Zaks, CMO at Moderna.
Moderna has also now finalized the Phase 3 study protocol based on feedback from the U.S. Food and Drug Administration (FDA). The randomized, 1:1 placebo-controlled trial is expected to include approximately 30,000 participants at the 100 µg dose level in the U.S. and is expected to be conducted in collaboration with NIAID, subject to regulatory approval.
With the Phase 3 dose at 100 μg, Moderna says it remains on track to be able to deliver approximately 500 million doses per year, and possibly up to 1 billion doses per year, beginning in 2021 due to collaborations with Lonza and Catalent.
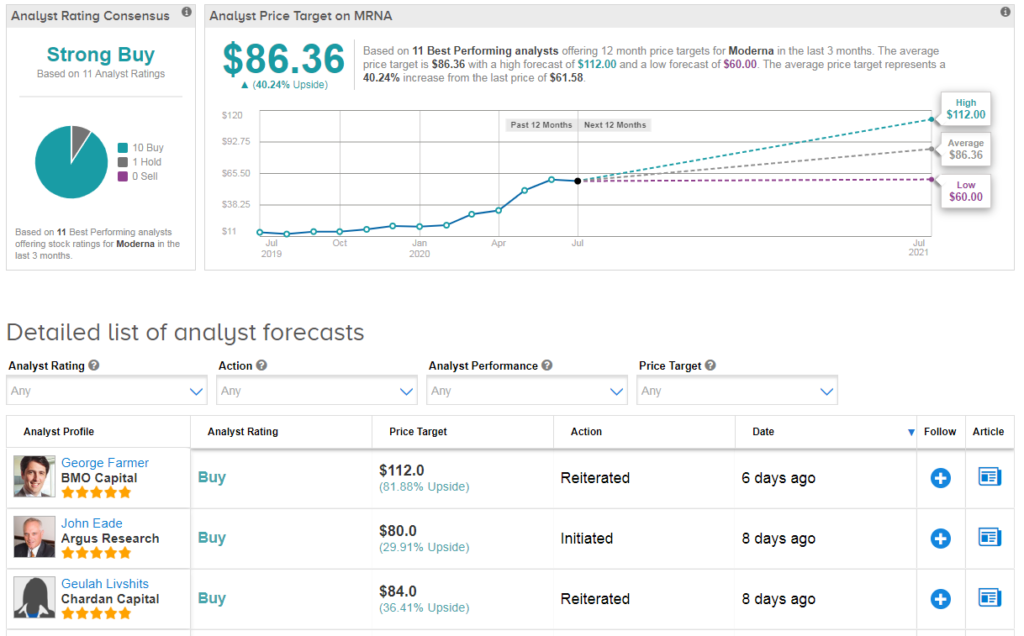
Enthusiasm over the vaccine has pushed the stock up 216% year-to-date, and analysts have a bullish Strong Buy consensus on Moderna’s outlook. Indeed, the average analyst price target of $86 suggests 39% further upside potential lies ahead. (See Moderna stock analysis).
“With Covid-19 cases rising across large swaths of the US, and a record high of new US cases we believe the stage is set for rapid evaluation of the study’s primary endpoint of symptomatic Covid-19 disease prevention” comments Chardan Capital analyst Geulah Livshits. She has a buy rating on the stock and $84 price target.
Meanwhile JP Morgan’s Cory Kasimov writes: “The company has spent almost a decade building a world-class platform around messenger RNA (mRNA) therapeutics, a new class of medicines that, if ultimately successful, could have broad and disruptive potential across the whole biopharma landscape.”
Related News:
Novavax Spikes 42% Pre-Market On $1.6B U.S. Funding For Covid-19 Candidate
Corvus Shoots Up 115% On Start Of Novel Immunotherapy Study In Covid-19 Patients
GenMark Soaring In Pre-Market On 118% Revenue Explosion









