Gilead Sciences (GILD) has announced encouraging additional data on remdesivir, the company’s investigational antiviral for the treatment of COVID-19.
The data includes a comparative analysis of the Phase 3 SIMPLE-Severe trial and a real-world retrospective cohort of patients with severe COVID-19.
“In this analysis, remdesivir was associated with an improvement in clinical recovery and a 62% reduction in the risk of mortality compared with standard of care – an important finding that requires confirmation in prospective clinical trials” the company stated.
Separate subgroup analyses from the Phase 3 SIMPLE-Severe trial, including an evaluation of the safety and efficacy of remdesivir across different racial and ethnic patient subgroups in the US, found that traditionally marginalized racial or ethnic groups treated with remdesivir experienced similar clinical outcomes as the overall patient population in the study.
Gilead also showed additional data on the company’s compassionate use program, which demonstrated that 83% of pediatric patients (n=77) and 92% of pregnant and postpartum women (n=86) with a broad spectrum of disease severity recovered by Day 28. No new safety signals were identified with remdesivir across these populations.
To further the understanding of these results in individual patient cases, Gilead has initiated a global, open-label Phase 2/3 trial to evaluate the safety, tolerability and pharmacokinetics of remdesivir in pediatric patients from birth to less than 18 years of age. Gilead is also collaborating on a study for pregnant women.
“We are working to broaden our understanding of the full utility of remdesivir. To address the urgency of the continuing pandemic, we are sharing data with the research community as quickly as possible with the goal of providing transparent and timely updates on new developments with remdesivir,” said Merdad Parsey, CMO of Gilead.
“These data presented at the Virtual COVID-19 Conference shed additional light on the use of remdesivir in specific patient populations, including those that may be susceptible to higher rates of COVID-19 infection, as well as others that are particularly vulnerable, including children and pregnant and postpartum women” he added.
Due to the current public health emergency, the U.S. Food and Drug Administration (FDA) has issued an Emergency Use Authorization for remdesivir for the treatment of hospitalized patients with severe COVID-19.
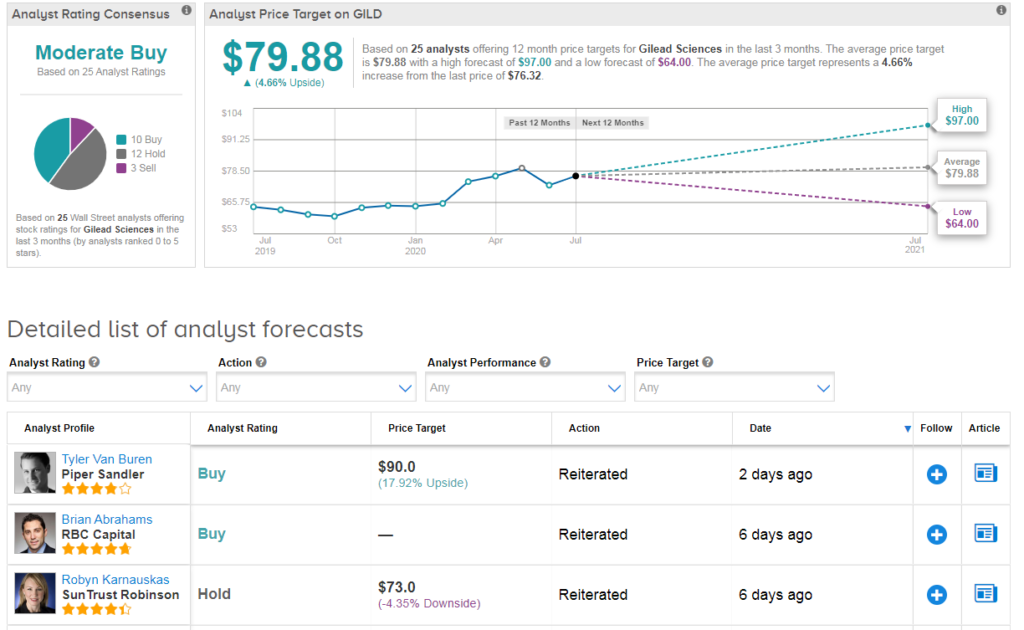
Five-star analyst Jim Birchenough at Wells Fargo recently reiterated a Hold rating on Gilead saying that he remains skeptical on the longer-term commercial opportunity in view of emerging competition and potential vaccine progress. Indeed, the Street is cautiously optimistic on the stock with a Moderate Buy consensus based on 10 Buy ratings, 12 Holds and 3 Sells.
Shares in Gilead rose 2% to $76.32 at the close of trading on July 10 taking the year-to-date advance to about 18%. The $80 average price target implies 5% upside potential in the shares in the coming 12 months. (See Gilead stock analysis on TipRanks).
Related News:
Novavax Spikes 42% Pre-Market On $1.6B U.S. Funding For Covid-19 Candidate
Gilead’s Covid-19 Remdesivir Therapy Gets Conditional European Nod
Moderna Inks Deal With Rovi To Supply Potential Covid-19 Vaccine Outside US









