French drugmaker Sanofi (SNYNF) has received regulatory approval in China for its Dupixent drug, which it is developing together with Regeneron Pharmaceuticals, Inc (REGN).
The two drugmakers said that China’s National Medical Products Administration (NMPA) has approved Dupixent for the treatment of moderate-to-severe atopic dermatitis in adults whose disease is not adequately controlled with topical prescription therapies or when those therapies are not advisable.
The announcement comes after Sanofi earlier this month said that it is marking the drug as one of its top growth drivers with expected peak sales of €10 billion.
“Sanofi has deep roots in China, and it continues to be a significant area of growth for us,” said Sanofi CEO Paul Hudson. “New regulations have paved the way for first-in-class treatments like Dupixent to be delivered to patients sooner and, in partnership with the government’s Healthy China 2030 initiative, we plan to seek approval by 2025 for more than 25 innovative medicines for chronic and rare diseases and vaccines.”
Moderate-to-severe atopic dermatitis is characterized by rashes that can potentially cover much of the body, and can include intense, persistent itching, skin dryness and skin lesions including cracking, redness or darkness, crusting and oozing. Dupixent is a fully-human monoclonal antibody that inhibits the signalling of the interleukin-4 (IL-4) and interleukin-13 (IL-13) proteins, and is not an immunosuppressant.
The approval was based on positive data from the global LIBERTY AD clinical trial program that included nearly 3,000 patients with inadequately controlled moderate-to-severe atopic dermatitis. The trials evaluated Dupixent on safety and efficacy measures, including skin clearance, overall disease severity and itch. Data from an ongoing Phase 3 trial in China of adults with moderate-to severe atopic dermatitis will be shared with NMPA in the second half of 2020 when the trial is expected to be completed.
Shares in Regeneron surged 7.8% to $643.80 on Friday boosting this year’s rally to an impressive 71%. Sanofi rose 1.7% to $103.50 as of the last close.
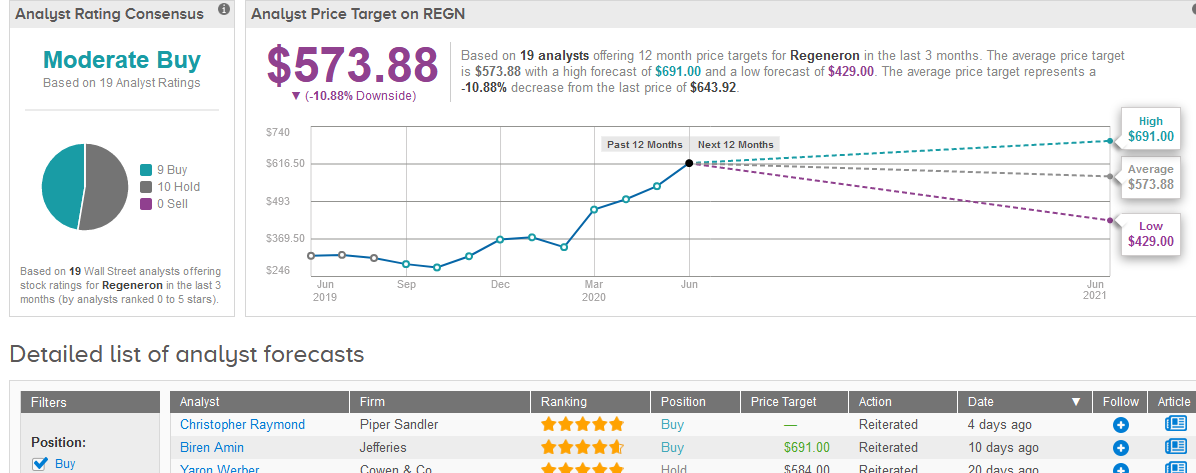
In light of Regeneron’s recent stock advance, the analysts’ $573.88 average price target now implies 11% downside potential in the coming 12 months. (See Regeneron stock analysis on TipRanks)
Five-star analyst Christopher Raymond last week reiterated a Buy rating on the stock, saying that he expects Dupixent to “remain the dominant player” in the atopic dermatitis space.
Overall, the 19 analysts covering the stock are divided between 9 Buy and 10 Hold ratings adding up to a Moderate Buy consensus.
Related News:
Regeneron Starts Human Clinical Trials Of Covid-19 Antibody Cocktail
Emergent Bio Signs Covid-19 Vaccine Manufacturing Deal With AstraZeneca
Oxford Biomedica Clinches Manufacturing Deal For AstraZeneca’s Covid-19 Vaccine