Shares in AstraZeneca (AZN) spiked almost 5% in after-market trading amid a report that early-stage human trial data on the drugmaker’s coronavirus vaccine candidate will be disclosed on July 20.
The stock rose to $60.60 in Wednesday’s extended trading after earlier jumping 7%. Data results from the Phase 1 trial of the potential coronavirus vaccine, also known as AZD1222, which AstraZeneca is developing with Oxford University, are slated to be published on Monday, according to the Lancet medical journal.
The data is expected to demonstrate whether the potential vaccine is safe and whether or not it triggers an immune response. Earlier this month, the vaccine developers said they were encouraged by the immune response they had seen in trials so far.
“We expect this paper, which is undergoing final editing and preparation, to be published on Monday, July 20, for immediate release,” a spokeswoman for the journal told Reuters.
Meanwhile, the vaccine candidate is already in large-scale Phase III human trials to assess whether it can protect against COVID-19.
AstraZeneca has in recent weeks signed supply chain agreements for the capacity to produce 2 billion doses of its vaccine candidate. The British drugmaker has inked supply deals with the U.S. and European Union countries.
AZD1222 is one of several candidates supported by Operation Warp Speed (OWS), the U.S. government program to accelerate the development, manufacturing, and distribution of COVID-19 vaccines available for Americans by Jan. 2021.
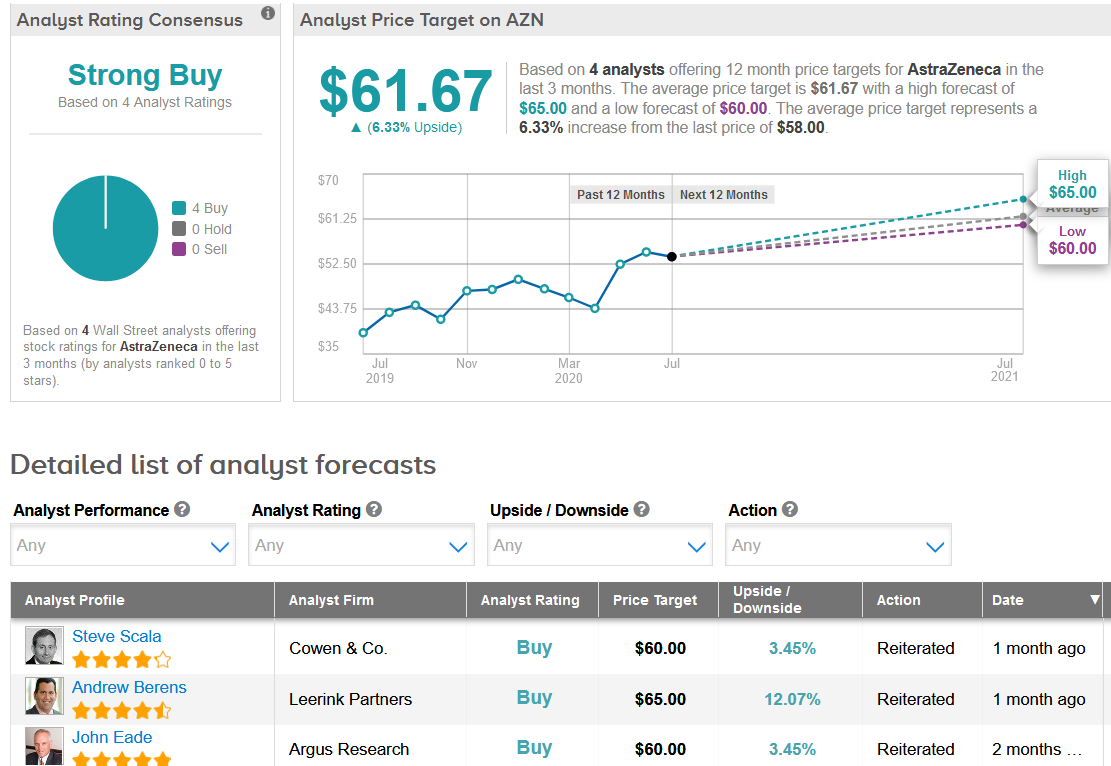
Shares have jumped 53% since mid-March as AstraZeneca joined the list of companies engaged in the development of a potential coronavirus vaccine. Despite the recent rally, the $61.67 average analyst price target still puts the upside potential at 6.3% in the coming 12 months. (See AstraZeneca stock analysis on TipRanks)
Overall, the stock scores a Strong Buy consensus from the analyst community based on 4 unanimous Buy ratings.
Related News:
AstraZeneca’s Wins FDA Priority Review For Heart Drug Brilinta
Novavax Spikes 42% Pre-Market On $1.6B U.S. Funding For Covid-19 Candidate
Is Moderna (MRNA) Stock Still Worth Buying After Its Huge Rally? JPMorgan Says Yes